Abstract
Introduction: Bone Marrow Transplant (BMT) represents the only cure for many malignant and non-malignant hematopoietic disorders. This treatment is often accompanied by multiple red blood cell transfusions, which can lead to iron overload. Iron overload is associated with poorer outcomes following BMT, such as increased risk of infections or Graft-versus-Host Disease (GVHD); however, it is not yet clear whether this correlation is caused by iron itself or is simply confounded by patients with more severe disease requiring more transfusions. Previous studies using mouse models, have shown that transfusional iron overload reduces the population of beneficial bacteria in the gut microbiome or decreases the production of microbiome-produced metabolites (i.e. indoles) that have been associated with BMT outcomes and GVHD risk. Here, we developed a reductionist mouse model of iron overload and BMT to investigate the effect of iron and iron-induced changes to gut microbiota and their metabolites on BMT outcomes and GVHD survival.
Methods: Female Balb/C mice were infused twice a week, for two weeks, with a total of 50 mg of iron dextran or saline followed by lethal irradiation (9 Gy). Cohorts of mice (n=10 per group) received 1x10^10 T cell-depleted bone marrow cells with or without 5x10^6 T cells from UBC-GFP C57Bl6 mice to establish allogenic BMT and GHVD models, respectively. Mice were then monitored daily for survival with a formal severity score evaluated twice a week. Analysis of donor red blood cells, platelets, and white blood cells (neutrophils, B and T cells) from peripheral blood was also performed once a week to check for engraftment. To examine the impact of iron-induced microbiota changes, fecal microbiome transplant (FMT) was performed in mice receiving BMT. Briefly, mice were treated with an antibiotic cocktail (ampicillin 1 g/L, kanamycin 1 g/L, metronidazole 1g/L, vancomycin 0.5g/L) by gavage 2 days before the start of FMT. Feces from control and iron overloaded mice were collected freshly and weighed. Stool pellets were then resuspended in PBS (100 mg feces/ml PBS), vortexed and then spun down at 800g for 3 minutes. Mice received 100ul of supernatant by gavage. Additional cohorts of mice were administrated with indole-3 proprionic acid (IPA) in the drinking water at a concentration of 0.1 mg/ml prior to irradiation throughout the experiment and monitored for survival after receipt of allogeneic T cells to induce GVHD. All mice received enrofloxacin (10mg/kg) starting on the day of BMT and for the following 3 days by intraperitoneal injection.
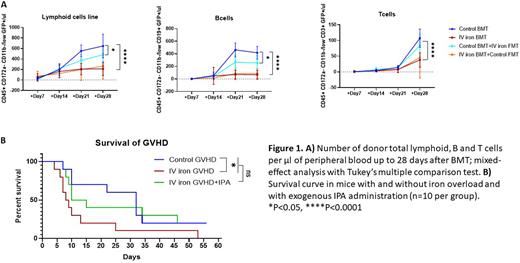
Results: There was a significant reduction in overall lymphoid (CD45+ CD172a- CD11b-/low GFP+), B (CD45+ CD172a- CD11b-/low CD19+ GFP+), and T (CD45+ CD172a- CD11b-/low CD3+ GFP+) cells in iron overloaded mice compared to control mice (p<0.0001 at day +28) by flow cytometry analysis in peripheral blood after BMT. Although FMT from control feces into iron overloaded mice did not affect lymphoid numbers, FMT from iron overloaded feces into control mice reduced the number of donor lymphoid and B cells (p<0.05 at day +28; Figure 1A).
Iron overload reduced survival compared to control mice in the GHVD mouse model (p<0.05 Mantel-Cox test). Administration of IPA in iron overloaded mice did not significantly affect overall survival (Figure 1B).
Conclusions: Our results show that iron overload negatively impacts lymphocyte engraftment in a mouse model of BMT. Furthermore, iron overload also reduces survival in mice with GVHD. Iron overload was previously shown to reduce indole levels in mice. Although we did not observe a significant impact of exogenous indole administration on GVHD survival, FMT from iron overloaded into control mice reduced levels of lymphoid and B cells after BMT implicating gut microbiota in this outcome. Taken together, these results suggest that iron overload is an important factor that negatively affects BMT outcomes. The extent to which iron-induced gut microbiota changes are involved in the mechanisms responsible for this effect requires further investigation.
Disclosures
Hudson:Alpine Immune Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal